PepGen raised $108 million in gross proceeds in its initial public offering, selling 9 million shares at $12 via BofA, SVB and Stifel Nicolaus.
The deal was raised in size from the proposed 7.2 million shares but lowered in price from the marketed range of $13 to $15, achieving just over the filed size, which would have generated $101 million at the midpoint of the range.
The company begins public life with a market capitalization of $268 million, and shares will begin trading Friday on the Nasdaq Global Select Market under symbol PEPG.
PepGen, based in Cambridge, Massachusetts, is developing therapies for serious genetic diseases, with an initial focus on neuromuscular diseases.
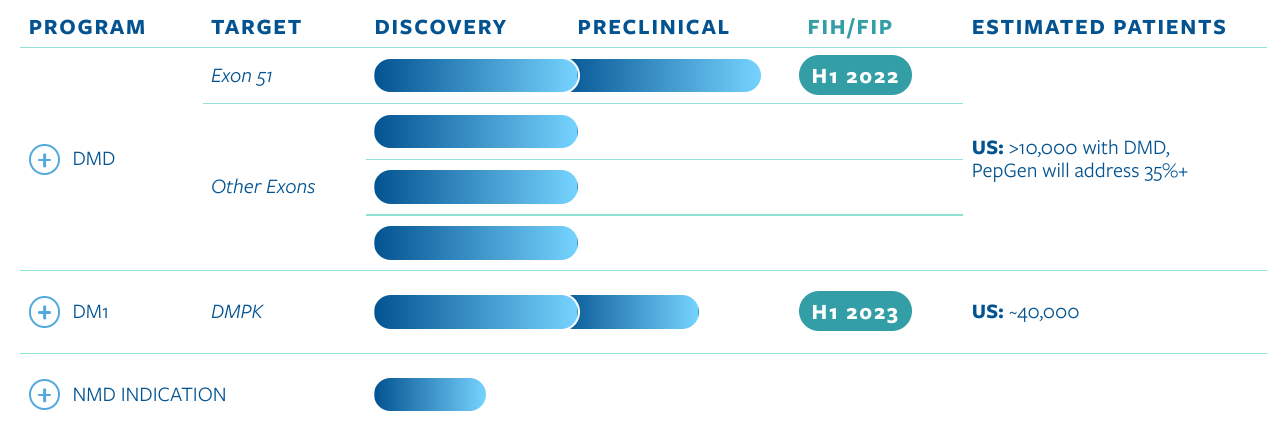
The company's initial target is Duchenne muscular dystrophy (DMD), a debilitating disease that predominantly affects males.
DMD results from genetic mutations in the gene that encodes dystrophin, a protein critical for healthy muscle function. While categorized as a rare disease, DMD is among the most prevalent with an incidence rate of about one in every 3,500 to 5,000 male births.
DMD is characterized by progressive muscle weakness, which leads to patients losing the ability to walk, a loss of upper body function, cardiac issues and difficulties breathing. DMD is invariably fatal by young adulthood.
PepGen's pipeline is based on its proprietary Enhanced Delivery Oligonucleotide (EDO) platform, which is designed to improve the activity and tolerability of therapeutics.
The company's EDO peptides have demonstrated the ability to transport oligonucleotides — short, single strands of DNA or RNA — across a broad range of target tissues, including smooth, skeletal and cardiac muscle, and the central nervous system.
Investment thesis
Like the large cap Bausch + Lomb, which also debuts Friday, PepGen had poor fortune with the timing of the IPO, given the market's significant swings.
We're encouraged, however, that, unlike Bausch + Lomb, PepGen was able to raise what it sought at just above its target market valuation.
Companies at this stage of development are difficult to gauge, of course, but we have reasons for optimism for this small cap biotech.
- The company was founded in 2018 with technology spun out from the University of Oxford and the Medical Research Council of United Kingdom Research and Innovation.
-
PepGen announced on April 6, 2022, that it had dosed its first patient in its Canadian Phase 1 trial of PGN-EDO51, the company’s lead product candidate for the treatment of DMD.
- Existing therapies are limited by poor delivery to muscle tissue and have yet to establish meaningful clinical benefit for DMD patients.
- Those who follow what the FDA has been doing with potential DMD therapies know that the agency is keen to approve any drug that shows even nominal benefit.
- If PepGen's EDO platform is successful in affecting DMD, we would expect the FDA to hasten the regulatory gauntlet.
- CEO James McArthur has 25 years of experience as a co-founder of numerous biotech companies focused on rare disease therapeutics.
- The company had $133 million in cash and equivalents as of Dec. 31, 2021. This deal adds about $100 million to that number, which is a big war chest for a company at this stage.
- That cash value also makes the stock a relatively inexpensive call option on the company's EDO platform.
_____
Source: Equities News



