Ligand Pharmaceuticals To Spin Off Antibody Discovery Business Via SPAC Merger
Ligand Pharmaceuticals ( LGND ) announced that it has reached an agreement with Avista Public Acquisition Corp II ( APAC ) by which Ligand will spin off its antibody discovery business, OmniAb, which will then immediately be merged into APAC.
Ligand had broadcast its intention in November 2021 to split into two public companies, separating the OmniAb from its royalties business and other technologies.
Shareholders of Ligand will receive 100% of OmniAb in a tax-free distribution and, post-merger, the combined company will have a valuation of $850 million. Avista Capital Partners, a leading private equity firm and APAC's sponsor, will invest up to $115 million into the new company.
Upon closing, Ligand shareholders are expected to own approximately 75% to 84% of the combined company, depending on redemptions. The new company will be listed on the Nasdaq Global Markets under the ticker symbol OABI.
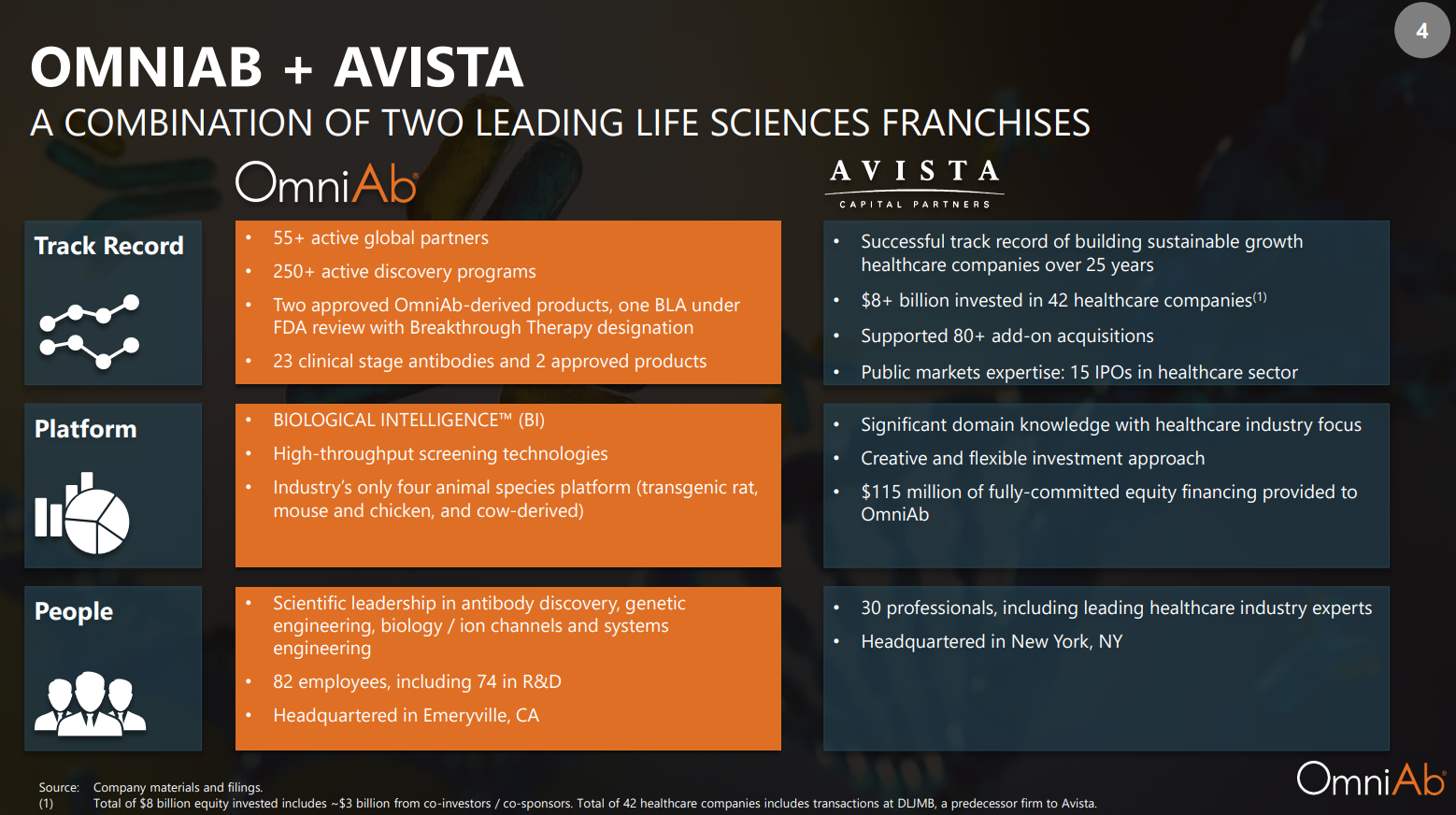
The OmniAb antibody discovery platform provides pharmaceutical companies with access to diverse antibody repertoires and high-throughput screening technologies to enable discovery of next-generation therapeutics.
Investment thesis
The spin off and merger are expected to close in the second half of 2022. Investors have a window until then to receive OmniAb as a tax-free distribution.
We think that the OmniAb antibody discovery business has greater potential for growth than what will stay behind with the legacy Ligand Pharmaceuticals.
- OmniAb has 13 years of investment, research and development behind it.
- Over 55 pharmaceutical partners currently have access to OmniAb-derived antibodies.
- Over 250 clinical programs are being actively developed or commercialized.
-
In 2021, two royalty-bearing antibodies received regulatory approvals in China.
-
In August, Gloria Biosciences received approval in China for zimberelimab (GLS-010), an OmniAb-derived fully human anti-PD-1 monoclonal antibody for the treatment of recurrent or refractory classical Hodgkin’s lymphoma.
- Zimberelimab is being developed by Arcus Biosciences in collaboration with Gilead in North America, Europe and Japan.
-
In December, CStone received approval in China for Cejemly (sugemalimab), an OmniAb-derived fully human anti-PD-L1 monoclonal antibody as part of first-line treatment for metastatic nonsquamous non-small cell lung cancer.
- It's the first and only anti-PD-L1 approved for first-line treatment in metastatic nonsquamous NSCLC anywhere in the world.
- Pfizer is responsible for commercializing Cehemly in China.
-
In August, Gloria Biosciences received approval in China for zimberelimab (GLS-010), an OmniAb-derived fully human anti-PD-1 monoclonal antibody for the treatment of recurrent or refractory classical Hodgkin’s lymphoma.
- Last year, nine antibodies derived from the OmniAb platform entered clinical testing.
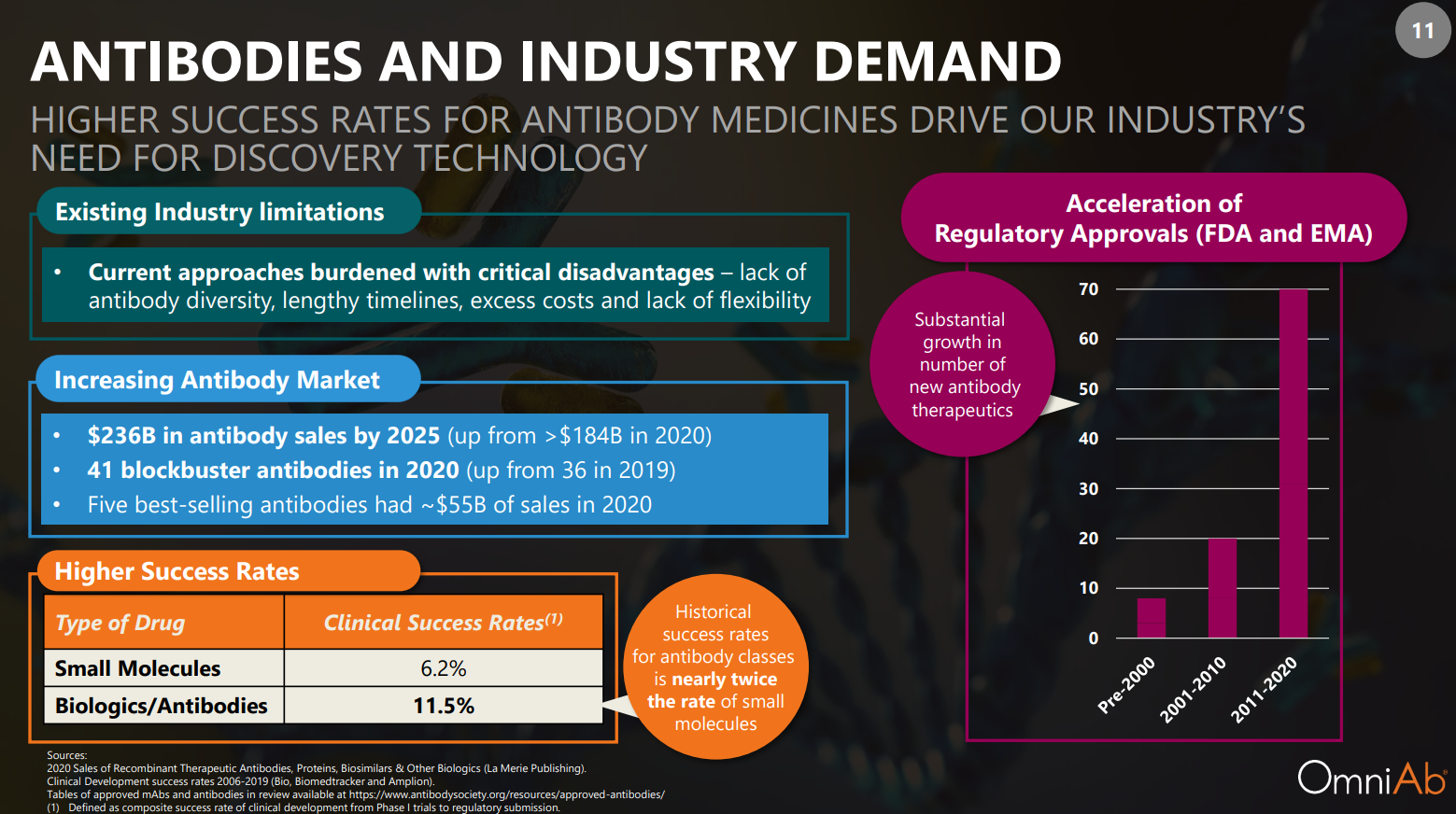
- The historical success rate for antibodies is nearly twice that of small molecules.
_____
Source: Equities News



