Aeglea BioTherapeutics Raises $45 Million Via Registered Direct Offering

Image source: Aeglea BioTherapeutics
Aeglea BioTherapeutics ( AGLE ) announced Thursday that it has raised $45 million in gross proceeds via a registered direct offering with institutional investors.
The deal comprises 10,752,688 shares of common stock at $1.60 per share and pre-funded warrants to purchase 17,372,397 shares of common stock at $1.5999 per warrant, which represents the per share offering price for the common stock less the $0.0001 exercise price.
Notably, the stock closed Wednesday at $1.58 per share, meaning the deal got priced at a premium to the market, a rare occurrence for registered directs.
Participating investors included Bain Capital Life Sciences, Great Point Partners, Nantahala Capital Management and Sio Capital Management.
Aeglea is developing human enzyme therapeutics as innovative solutions for rare metabolic diseases. The company's lead candidate is pegzilarginase for patients with Arginase 1 deficiency (ARGD-1), a rare, progressive disease characterized by high levels of arginine, an amino acid that helps the body build proteins.
People, primarily children, living with ARG1-D experience severe spasticity-related mobility limitations, seizures, developmental delay, intellectual disability and early mortality.
The company announced on April 12, 2022, that it had filed its first Biologics License Application with the FDA for pegzilarginase, accompanied by a request for Priority Review.
Pipeline
Beyond pegzilarginase for ARG1-D, Aeglea is in Phase 1 studies with AGLE-177 for homocystinuria, a genetic disorder in which the body is unable to process the amino acid methionine.
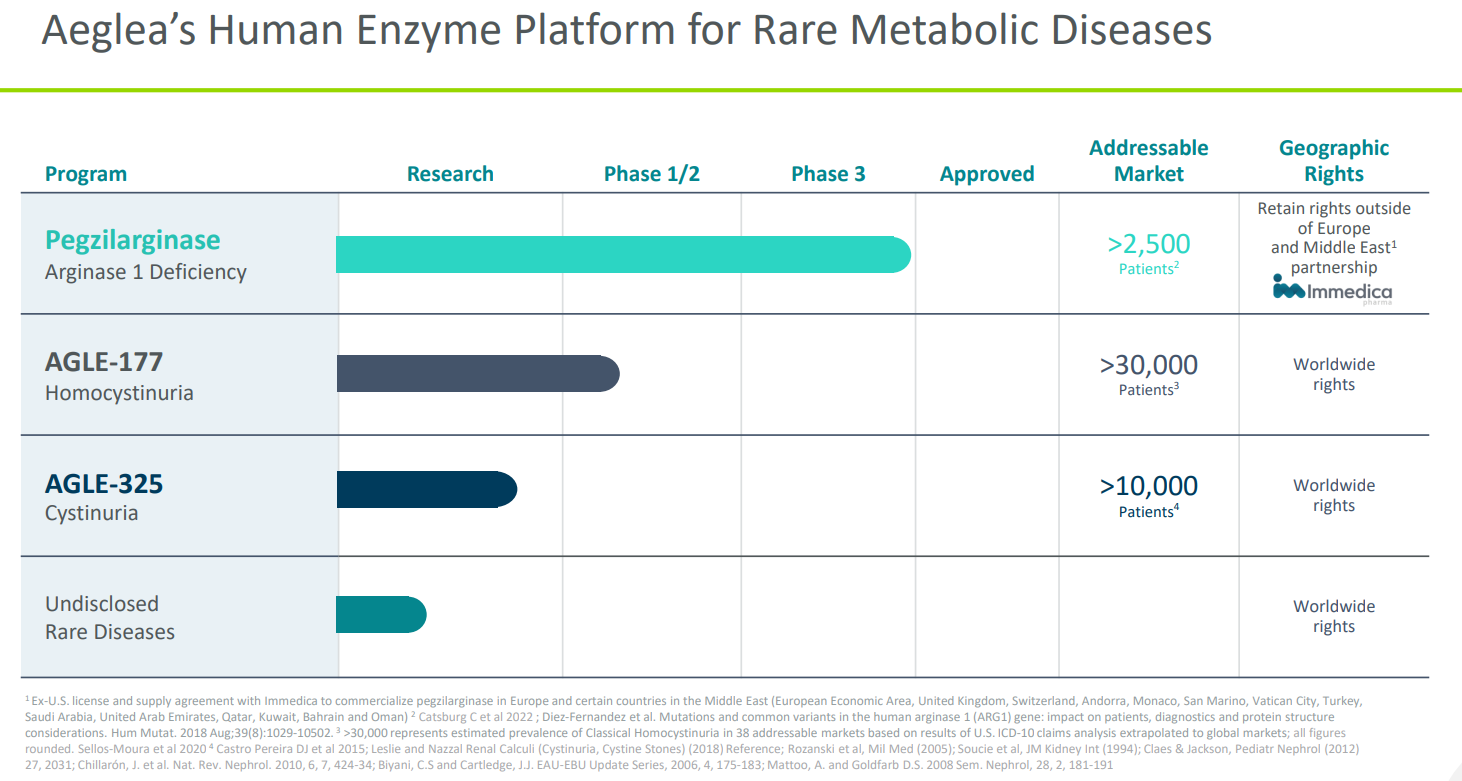
Image source: Aeglea BioTherapeutics
Investment thesis
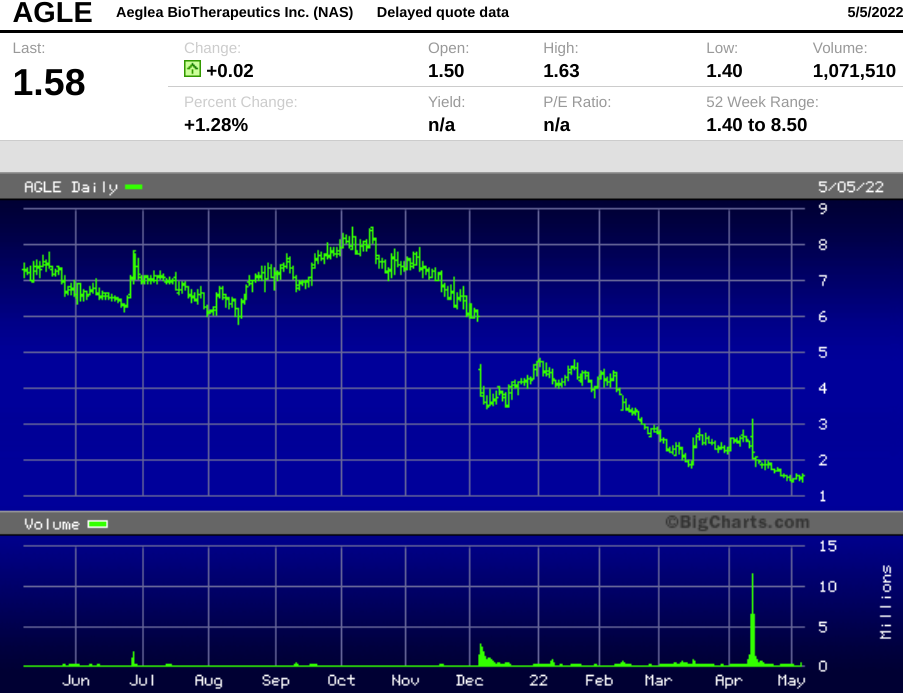
Investors punished Aeglea in December 2021, when it released data from its Phase 3 trial of pegzilarginase for ARG1-D.
Despite the fact that the company achieved the primary endpoint of the study, showing that the drug achieved 80% reduction in plasma arginine, investors were disappointed with the secondary endpoint of improving mobility.
The data showed that pegzilarginase did improve mobility vs placebo, but the difference was not statistically significant.
We think investors overreacted in December, but the stock hasn't recovered.
We feel that investors with a high tolerance for risk may want to examine Aeglea at current levels as essentially a call option on the FDA's approval of pegzilarginase:
-
This $45 million provides a significant boost to the $68.6 million in cash and equivalents that Aeglea had as of March 31, 2022.
- The new total should provide runway into the second half of 2023.
- There are no approved therapies for ARG1-D.
-
Pegzilarginase has received multiple regulatory designations from the FDA, including:
- Rare Pediatric Disease
- Breakthrough Therapy
- Fast Track
- Orphan Drug
- It has also received Orphan Drug Designation from the European Medicines Agency.
- We think Aeglea has a good chance of getting FDA approval given the Phase 3 results.
_____
Source: Equities News



